-
Equipment and Materials
-
Equipment -
COVID-19 antigen rapid diagnostic test kit
-
Materials -
Gloves
-
Prior to Performing Test
-
Prescriber shall place the order in the Integrated Behavioral Health Information System (IBHIS) prior to authorized personnel performing the test.
-
Authorized personnel shall review the order in IBHIS.
-
Authorized personnel shall ask client to state or provide at least two (2) unique identifiers. Unique identifiers can be any combination of the following:
• Last Name, First Name
• Date of Birth (DOB)
• IBHIS Number
• Last 4 digits of Social Security Number (SSN)
• Address
• Phone Number
-
Obtain the following information from client and document in IBHIS:
a. Preferred method of notification of test results
b. Current COVID-19 symptoms, if any. Please also document if client reports no COVID-19 symptoms.
5. Follow universal precautions.
-
Explain procedures to the client and provide the necessary supplies, including a label with their full name and IBHIS number on the COVID-19 antigen rapid diagnostic test kit.
-
Specimen Collection
-
Samples shall be collected from clients within the first five (5) days of symptom onset, from clients without symptoms, or from clients who have other epidemiological reasons to suspect COVID-19 when tested twice over two (2) or three (3) days with at least 24 hours and no more than 48 hours between tests.
-
Nasopharyngeal Swab Collection -
Remove the nasopharyngeal swab from the pouch. -
Tilt the client's head back 70 degrees. -
Gently and slowly insert the swab into one of the nostrils until it reaches the posterior nasopharynx. -
Keep inserted until resistance is encountered or the distance is equivalent to that from the ear to the client's nostril. -
Slowly rotate the swab three (3) to five (5) times over the surface of the posterior nasopharynx. -
Leave the swab in place for several seconds to absorb secretions. -
Slowly remove the swab from the nostril while rotating it.
-
Anterior Nasal Swab Collection -
Remove the nasopharyngeal swab from the pouch. -
Insert the swab into one (1) of the nostrils up to one (1) inch from the edge of the nostril. -
Slowly roll the swab over the surface of the nostril five (5) times. -
Using the same swab, repeat this collection process in the other nostril. -
Collect specimen over approximately 15 seconds. -
Slowly remove the swab from the nostril while rotating it.
-
Test Procedure
-
Allow test devices, reagents, specimens, and/or controls to equilibrate to room temperature prior to testing.
-
Remove the COVID-19 antigen test device and extraction vial from its foil pouch immediately before testing.
-
Peel off the aluminum foil seal from the top of the extraction vial containing the extraction buffer.
-
Place the swab into the extraction vial and swab vigorously at least five (5) times.
-
Remove the swab by rotating against the extraction vial while squeezing the sides of the vial to release the liquid from the swab.
-
Properly discard the swab.
-
Close the vial with the provided cap and push firmly onto the vial.
-
Mix thoroughly by flicking the bottom of the tube.
-
Invert the extraction vial and hold the sample vertically above the sample well.
-
Squeeze the vial gently and allow three (3) drops of sample to fall into the sample well. -
Two (2) drops of the sample are the required minimum volume to initiate the test run. -
Invalid results will be obtained if one (1) drop of sample is added to the cassette. -
Leakage of the sample is possible when six (6) drops or more of the sample are added.
-
Read and interpret the test result at 10 minutes. -
The test result should not be read and interpreted after 15 minutes.
-
Quality Control
-
Run positive and negative external control swab provided in the kit with every new lot.
-
Contact manufacturer if external control results are invalid.
-
Result Interpretation
-
Negative result -
One red-colored line only next to “C” indicates a negative result. 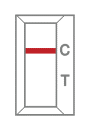 -
Positive result -
Two (2) distinct colored lines appear. One red-colored line next to “C” and one blue-colored line next to “T” indicate a COVID-19 positive result. Any faint colored line(s) in the test region(s) should be considered positive. 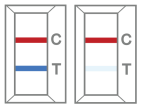 -
Invalid result -
If the red-colored line in the control region “C” ” is not visible, the result is invalid. Re-run the test one time using the remaining specimen in the extraction vial if an invalid result is obtained during initial testing. 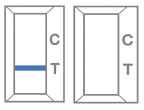 -
False Positive Test -
May occur if the test is interpreted outside of the interpretation window.
-
False Negative Test -
May occur if the test is interpreted outside of the interpretation window. -
Incorrect specimen collection method. -
Inadequate specimen collection. -
Improper specimen handling and/or transport.
-
Documentation -
Authorized personnel shall document results in IBHIS as a point-of-care COVID-19 antigen test result.
- Report Test Results
- Authorized personnel shall report positive test results to the Department of Public Health (DPH) via www.SimpleReport.gov. Negative test results are not required to be reported to DPH.
- Reporting positive test results via SimpleReport.
- Log in to SimpleReport.
- Select DMH site / clinic.
- Click on Conduct Tests tab.
- Enter name of client.
- Select correct client based on full name and date of birth.
- If client not yet added to the system, click Patients tab, +add patient, and enter client’s information before saving changes.
- Click Begin Test.
- Complete Patient Questionnaire, which includes:
- Method client would like to be notified of test results.
- Symptoms client reported at time of test.
- Select testing device and swab type from dropdown menus.
- Select result of COVID-19 test.
- Click submit once all questions have been completed.
| |
|