-
Equipment - Rapid chromatographic immunoassay test cassette for qualitative determination of human chorionic gonadotropin (hCG) in urine
- Test cassettes shall be stored between 4°C to 30°C (40°F to 86°F).
- Authorized personnel shall check the expiration date of a test cassette prior to use. Open or expired test cassettes shall not be used.
- Transfer pipette (inside test cassette)
- Disposable, clean, dry plastic container for urine specimen collection
- Materials
- Prior to Performing Test
- The prescriber shall place an order in IBHIS prior to the Authorized Personnel performing the test.
- Authorized Personnel shall review the order in IBHIS.
- Authorized Personnel shall ask client to state or provide at least two (2) unique identifiers. Unique identifiers can be any combination of the following:
- Last Name, First Name
- Date of Birth (DOB)
- IBHIS Number
- Last 4 digits of Social Security Number (SSN)
- Address
- Phone Number
- Authorized Personnel shall follow universal precautions.
- Authorized Personnel shall explain procedures to the client and provide necessary supplies, including a label with the client's full name and IBHIS number on the urine collection container.
- Performing Test
- Specimen Collection
- Urine is to be collected in a clean, dry, plastic container without preservatives. Urine collection cup provided by DMH Pharmacy.
- Urine specimens collected at random may be used; however, first-morning urine specimen is preferred.
- Testing shall be performed immediately without refrigeration
- Instruct client to
- Remove cap from test cup.
- Void directly into test cup.
- Attach and tighten lid.
- Return collection cup with urine specimen to Authorized Personnel.
- Wash hands and put on gloves.
- Obtain the urine specimen from the client in an appropriately labeled container.
- Remove the test cassette and pipette from the pouch and lay them on a clean, level surface.
- Label the test cassette with the client's identifying information.
- Holding the supplied pipette at a slight angle, authorized personnel shall:
- Squeeze the bulb
- Draw enough sample to fill the stem completely
- Slowly discharge three drops of the urine sample from the pipette stem into the sample well of the cassette being careful not to overfill the absorbent pad.
- Wait for the pink-purple line(s) to appear. Read results at five (5) minutes.
- Interpret result.
- Empty the urine cup by disposing of urine in the toilet.
- Remove label with client's identifying information and properly dispose label in accordance with HIPAA regulations
- Discard used test cassette, pipette, and empty urine cup in the regular trash
- Remove gloves and wash hands.
- Result Interpretation
- Caution
- A low hCG concentration might result in a weak line appearing in the test region (T) after an extended period of time; therefore, DO NOT INTERPRET RESULTS AFTER 10 MINUTES.
- Limitations
-
Occasionally specimens containing less than 25 mIU/mL of urine also yield positive results.
-
In addition to pregnancy, hCG has been found in clients with both gestational and non-gestational trophoblastic disease. Since the hCG has been found in clients with both gestational and non-gestational neoplasms, similar to that found in pregnancy, these conditions, which include choriocarcinoma and hydatichiform mole, should be ruled out before a diagnosis of pregnancy is reached.
-
A normal pregnancy can not be distinguished from an ectopic pregnancy based on hCG levels alone. Also, spontaneous miscarriage may cause confusion in interpreting test results.
-
A very early pregnancy containing an extremely low concentration of hCG can give a negative result. In this case, another specimen should be obtained and tested at least 48 hours later.
-
hCG levels may remain detectable for several weeks after normal delivery, delivery by cesarean section, spontaneous abortion, or therapeutic abortion.
-
Negative result
-
Only one pink-purple colored band appears in the control area (C) as shown in Figure 1. 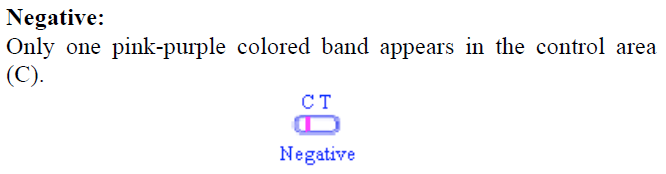 Figure 1. Negative Result Figure 1. Negative Result -
Positive result
-
In addition to the control band, a clearly distinguishable pink-purple colored band also appears in the test area (T) as shown in Figure 2. 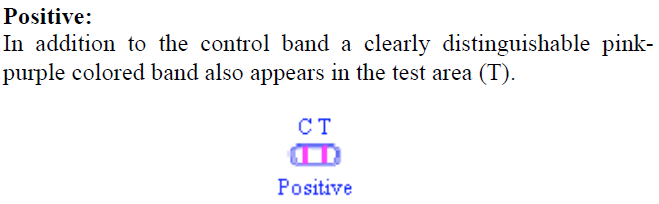 Figure 2. Positive Result Figure 2. Positive Result -
Invalid result
-
As long as there is no distinct pink-purple colored band visible in the control area, the test is invalid as shown in Figure 3. It is recommended that in this case, the test be repeated or a fresh specimen be obtained and tested 48 hours later. 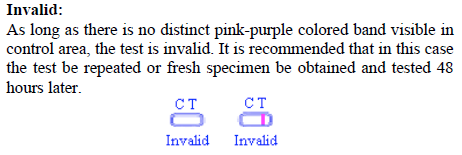 Figure 3. Invalid Result Figure 3. Invalid Result -
Quality Control
-
After the addition of a urine sample and upon test completion, a pink-purple colored band in the “C” area of the test cassette on negative samples and a pink-purple colored band in the “T” and “C” area on positive samples should appear. The appearance of the CONTROL band indicates that the test cassette is performing properly and serves as procedural control.
-
Documentation
-
Authorized personnel who perform and interpret test results shall document point-of-care pregnancy test results in IBHIS. | | | |
|