- Equipment and Materials
- Equipment
- Rapid chromatographic immunoassay test kit for qualitative determination of various drugs in urine
- Material
- Gloves
- Label for client identifiers
- Security seal, if applicable
- Prior to Performing Test
- Prescriber shall place order in IBHIS prior to Authorized Personnel performing the test.
- Authorized Personnel shall review the order in IBHIS.
- Authorized Personnel shall ask client to state or provide at least two (2) unique identifiers. Unique identifiers can be any combination of the following:
- Last Name, First Name
- Date of Birth (DOB)
- IBHIS Number
- Last 4 digits of Social Security Number (SSN)
- Address
- Phone Number
- Authorized Personnel shall follow universal precautions.
- Authorized Personnel shall remove test cup from pouch by tearing at the notch and remove desiccant pouch from cup.
- Authorized Personnel shall write client's full name and IBHIS number on a separate label, provided by DMH Pharmacy, and apply on top of lid of urine test cup.
- Authorized Personnel shall explain procedures to client and provide necessary supplies. Authorized Personnel shall check expiration date of test kit prior to providing to client.
- Performing Test
- Urine shall be collected in the test cup provided.
- Collect urine as soon as possible.
- Instruct client to
- Remove cap from the test cup
- Void directly into the test cup
- Fill cup to 30 mL mark
- Attach and tighten lid
- Immediately return cup with urine specimen to Authorized Personnel
- Wash hands and put on gloves.
- Obtain urine specimen from the client in appropriately labeled container.
- Ensure cap is screwed tight and place the cup on a flat surface.
- Read the temperature between 2 to 4 minutes after urine is voided into the cup to verify that the urine temperature is within the acceptable range 32°C to 38°C (90°F to 100°F). If results are outside of acceptable range, do not read drug test results and instruct client to provide urine specimen again with a new test cup.
- Peel off product label from right to left to reveal test results. If applicable, read adulteration results immediately. Verify adulteration pad colors are within acceptable range according to the manufacturer's adulteration color comparison chart. If results indicate adulteration, do not read the drug test results. Instruct client to provide urine specimen again with a new test cup.
- Read result at five (5) minutes.
- Interpret result.
- Remove label with client's identifying information and properly dispose label in accordance to HIPAA regulations.
- Empty urine cup by disposing urine in toilet.
- Discard used empty urine cup in regular trash.
- Remove gloves and wash hands.
- Quality Control
- Verify that temperature strip colors are within acceptable range
- Temperatures within 32°C (90° F) and 38°C (100° F) are considered within acceptable range. If results are outside of accepted temperature range, urine specimen may be altered (e.g. adulterated or diluted) or substituted.
- Verify that adulterant pad colors are within acceptable range
- Creatinine: indicator forms a purplish-brown color complex when creatinine is present at a normal level. The indicator will appear as a very light or no pad color change if creatinine concentration is less than 20 mg/dL, which indicates adulteration in the form of specimen dilution.
- Glutaraldehyde: presence in urine sample indicates the possibility of adulteration. However, a false positive may result when ketone bodies are present in urine due to ketoacidosis, starvation, or other metabolic abnormalities.
- Nitrite: levels above 15 mg/dl are indicative of adulteration.
- Oxidant/Bleach: indicator will form a blue-green, medium to dark brown, or orange color, if urine specimen is adulterated with bleach or other oxidizing agents.
- pH: Normal urine pH ranges from 4.5 to 8.0. Values below pH 4.0 or above pH 9.0 are indicative of adulteration.
- Specific gravity: indicator will change from dark blue to blue-green in urine of low ionic concentration to green and yellow-green in urine of higher ionic concentration. Specific gravity below 1.003 or above 1.025 is considered abnormal.
- Wait five (5) minutes to determine a positive result. DO NOT READ results after five (5) minutes. Results after more than five (5) minutes may be inaccurate and should not be read.
-
Result Interpretation
-
Limitations:
-
This test has been developed for testing urine samples only. No other fluids have been evaluated. DO NOT use this device to test substances other than urine.
-
Adulterated urine samples may produce erroneous results. Strong oxidizing agents such as bleach (hypochlorite) can oxidize drug analytes. If a sample is suspected of being adulterated, obtain a new sample in a different, unused cup.
-
This test is a qualitative screening assay. It is not designed to determine the quantitative concentration of drugs or the level of intoxication.
-
Note:
-
There is no meaning attributed to line color intensity or width. Any visible line is considered to be a line.
-
Certain lines may appear lighter or thinner than other lines. ANY COLORED LINE VISIBLE IN THE TEST “T” REGION, NO MATTER HOW DARK OR FAINT, SHOULD BE INTERPRETED AS A NEGATIVE RESULT. (IMPORTANT: This assay provides only a preliminary analytical test result. A more specific alternative chemical method must be used in order to obtain a confirmed analytical result. Gas Chromatography/Mass Spectrometry (GC/MS) is the preferred confirmatory method. Clinical consideration and professional judgment should be applied to any drug test result, particularly when preliminary positive results are indicated.)
-
Negative result:
If a rose-pink band is visible in each control region, it indicates that the concentration of the corresponding drug of that specific test zone is absent or below the detection limit of the test.
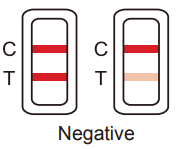
Figure 1. Negative Result -
Preliminary Positive result: A rose-pink band is visible in each control region. If no color band appears in the appropriate test “T” region, a preliminary positive result is indicated for the corresponding drug of that specific test zone. If lab confirmation is desired, apply security seal included in the urine drug screen test packaging to seal collection cup containing preliminary positive specimen and send for lab confirmation.
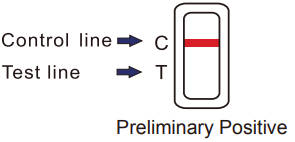
Figure 2. Preliminary Positive Result -
Invalid result:
If a color band is not visible in the control “C” region or a color band is only visible in the test “T” region, the test is invalid. Another test should be opened and run to re-evaluate the specimen. If test still provides an invalid result, please contact DMH Pharmacy office and provide the lot number of the test.
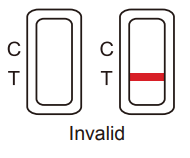
Figure 3. Invalid Result -
False Positive Test
-
Most common cause of a false positive test are cross reactants. Certain goods, medicines, diet plan drugs, and nutritional supplements may cause a false positive test result with the product.
-
False Negative Test
-
Documentation:
-
Authorized Personnel shall document result in IBHIS as point-of-care Urine Drug Test result. | | | |
|