UAB Enterprise Conflict of Interest Conflict of Commitment
| Review/Revised Date: 01/01/2021 Category: Ethics and Integrity
Policy Owner: Compliance and Risk Assurance
| |
TABLE OF CONTENTS
- General Roles and Responsibilities
- External Activities
- Internal Activities
- Professional Public Service Activities
- Outside Faculty Appointments
- Investigator Responsibilities
- Institutional Responsibilities for Investigator Conflicts of Interest
- Institutional Responsibilities for Institutional Conflicts of Interest
Definitions of Italicized Terms
This policy applies to all UAB ENTERPRISE employees. It also applies to investigators, including but not limited to students, trainees, visitors, and volunteers, who engage in research on behalf of UAB ENTERPRISE.
However, this policy does not displace or supplant any obligations of a “senior administrator” under Board Rule 106 of the Board of Trustees of the University of Alabama, or any obligations of any UAB ENTERPRISE employee under the Alabama Ethics Law, Ala. Code § 36-25-1, et. seq.
UAB ENTERPRISE is committed to conducting its activities in ways that promote and maintain public trust. UAB ENTERPRISE encourages professional interactions, activities, and development that enhance an employee's value to UAB ENTERPRISE; that enhance UAB ENTERPRISE's presence in the local, national, or international communities; and that provide public service. Nonetheless, in the complex environment of a major urban university and academic medical center, these opportunities may also present potential for, or appearance of, conflicting loyalties and responsibilities.
The UAB Enterprise Code of Conduct sets forth the expectation that employees be aware of the potential for conflicts of interest and conflicts of commitment and exercise due care to identify, review, and manage those conflicts appropriately. The purpose of this policy is to outline specific requirements for transparency and objectivity to help assure the integrity of academic and administrative endeavors and to protect UAB ENTERPRISE, its community members, and the public. It serves to communicate UAB ENTERPRISE’S overarching position on conflicts of interest and commitment. Other applicable policies or procedures may be adopted by UAB ENTERPRISE organizations, schools, or departments to supplement this policy.
- GENERAL ROLES AND RESPONSIBILITIES
UAB ENTERPRISE employees are required to:
- avoid engaging in activities, decisions, or actions, occurring anywhere or anytime, in which they, their families, or their businesses will gain or could be perceived to gain financially or personally because of the employee’s position at UAB ENTERPRISE unless conflicts of interest and/or commitment are appropriately managed as outlined in this policy (Section E.);
- obtain approval prior to engaging in external or internal activities in accordance with this policy and the procedures of the UAB ENTERPRISE organization with which they are primarily employed;
- properly account for time away engaged in external or professional public service activities;
- avoid use of UAB ENTERPRISE information and resources (e.g., facilities, personnel, equipment, and intellectual property) in external activities, unless such use is approved by the UAB Enterprise in writing and reimbursement to the UAB ENTERPRISE is made at fair market value for any use of information and resources that exceeds thresholds allowed by law or policy (see Use of UAB Resources by External Entities and Acceptable Use of Computer and Network Resources);
- notify unit leadership of any personal or financial relationships that relate to their institutional responsibilities in accordance with this policy and the procedures of the UAB ENTERPRISE organization with which they are primarily employed;
- adhere to any conflict of interest or conflict of commitment management or monitoring plan prescribed by their leadership; and
- report good faith concerns about undisclosed or unmanaged conflicts of interest or commitment through appropriate channels as described in the UAB Enterprise Code of Conduct and UAB’s Duty to Report and Non-retaliation Policy
Full-time faculty and institutional officials are required to:
- abide by the requirements for employees outlined above; and
- disclose financial interests related to their institutional responsibilities.
Investigators are required to:
- abide by the requirements for employees outlined above;
- complete required training on financial conflicts of interest, as applicable;
- disclose financial interests related to their institutional responsibilities; and
- ensure all applicable external, internal, or professional public service activities and/or financial interests are appropriately disclosed in Other Support, Biosketch, and/or Foreign Component sections of federal agency grant applications, just-in-time submissions, and/or progress reports for any project for which the investigator serves as the principal investigator (PI), co-PI, or any role in which the investigator has been named key personnel.
Deans, chairs, and other supervisors are required to:
- administer this policy;
- ensure adequate controls are in place to review and approve external activity requests submitted by employees in their units, such that the activity:
- does not interfere with, or appear to interfere with, the employee’s institutional responsibilities (i.e., their primary obligations to UAB ENTERPRISE);
- is compatible with the interests of UAB ENTERPRISE as a public academic institution; and
- does not violate state law and policy related to use of UAB ENTERPRISE resources or facilities;
- ensure adequate controls are in place to review and approve internal activity requests submitted by employees in their units, such that the activity:
- does not interfere with, or appear to interfere with, the employee’s institutional responsibilities (i.e., their primary obligations to UAB ENTERPRISE);
- is related to specialized training or knowledge the employee has, which is essential to the project; and
- is above and beyond the commitments of the employee’s position;
- ensure proper accounting of employees’ time away for purposes of external, internal, and professional public service activities;
- ensure use of UAB ENTERPRISE information and resources, including facilities, personnel, equipment, patents, copyrights, technology, and work product in approved external activities is contracted for and approved in writing by UAB Enterprise and reimbursement is made to UAB ENTERPRISE at fair market value for such use where the use exceeds thresholds allowed by law or policy;
- ensure written financial arrangements among the affected UAB Enterprise organizations are in place for payment of the portions of employees’ salaries attributable to shared appointments/assignments/work projects performed for more than one UAB ENTERPRISE organization (e.g., employee lease agreements);
- develop, implement, and/or monitor plans for managing conflicts of interest and conflicts of commitment within their units, including reasonable protections for any student, fellow, or trainee involved in conflict of interest or conflict of commitment situations; and
- adopt specific procedures or more restrictive standards, if desired for their units.
B. EXTERNAL, INTERNAL, AND PROFESSIONAL PUBLIC SERVICE ACTIVITIES
UAB ENTERPRISE employees’ participation in external, internal, and professional public service activities is encouraged and has the potential to enhance UAB ENTERPRISE’S mission, bring esteem to UAB ENTERPRISE, advance progress in science, medicine, and the arts, and translate highest caliber academic expertise to practice. Nonetheless, employees are expected to devote their primary professional loyalty, time, and energy to their position at UAB ENTERPRISE. Employees must ensure that their external, internal, and professional public service activities:
- do not require absence as to cause the employee to neglect primary teaching, research, patient care, business obligations, or become regularly unavailable to students and/or colleagues;
- do not obligate the employee in such a manner as to violate prior, superseding obligations to UAB ENTERPRISE;
- are conducted in accordance with UAB ENTERPRISE policies related to disclosure of discoveries and inventions, patents, and use of computer software; and
- do not use the name or logos of any UAB ENTERPRISE entity in any such manner as to indicate that it is participating in, or in any way is sponsoring, a non- UAB ENTERPRISE activity.
Therefore, any perceived, potential, or actual conflicts of interest or conflicts of commitment with the employee’s primary institutional responsibilities caused by such activities must be identified, reviewed, and managed in accordance with this policy. It is the responsibility of unit leadership to exercise judicious oversight and control of external, internal, and professional public service activities so that no UAB ENTERPRISE functions or policies are compromised. It is possible to have a conflict of interest or conflict of commitment even with an uncompensated activity. For example, even if an employee receives no payment, multiple presentations to multiple different outside organizations could require so much of an employee’s time as to rise to the level of a conflict of commitment with primary teaching, research, or service responsibilities. It is also possible to have a conflict of interest or commitment outside of normal working hours, during periods of non-service (e.g., summer term for faculty having a 9-month appointment, weekends, etc.), or during leave of any type.
Employees must:
- request and receive approval of external activities in advance of performance, every 12 months (if the external activity is ongoing), and each and every time material changes in the nature of the activity, time committed, or remuneration received occur.
- University employees and investigators (regardless of title, position, or employment) who wish to engage in an external activity must complete a Request for External Activity Approval Form.
- All other UAB ENTERPRISE organizations will designate a process for their employees to obtain approval for external activities in accordance with the standards in this policy.
- properly account for time spent on external activities in accordance with applicable UAB ENTERPRISE human resource policies for applying leave time, as well as maximum time allowed.
The decision whether to approve an external activity and any associated leave request is at the discretion of and resides with deans, chairs, and supervisors.
Employees must:
- Request and receive approval of internal activities in advance of performance and each and every time material changes in the nature of the activity, time committed, or remuneration received occur.
- University employees who wish to engage in an internal activity must complete a Request for Internal Activity Approval Form.
- All other UAB ENTERPRISE organizations will designate a process for other employees to obtain approval for internal activities in accordance with the standards in this policy.
The decision whether to approve an internal activity is at the discretion of and resides with deans, chairs, and supervisors.
Professional public service activities, whether or not compensated, do not require advance approval unless otherwise directed by college/school or department leadership.
However, employees must:
- properly account for time spent on professional public service activities in accordance with applicable UAB ENTERPRISE human resource policies for applying leave time, as well as maximum time allowed.
- register any international travel associated with performance of a professional public service activity through the UAB International Travel Registration Form in accordance with the UAB-Related International Travel Policy.
In addition, investigators and institutional officials must:
- Submit a Notice of Professional Public Service Activity Form for any resulting financial interest (which includes sponsored or reimbursed travel) exceeding $5,000 acquired from any one entity in the previous 12 months for their participation in professional public service activities;
- Exception: investigators and institutional officials do not have to submit such form for such financial interest if the financial interest is acquired in return for providing seminars, lectures, presentations, or service on advisory committees or review panels for U.S. federal, state, or local government agencies, U.S. institutions of higher education as defined at 20 U.S.C. 1001(a), U.S. academic teaching hospitals, U.S. medical centers, or U.S. research institutes that are affiliated with a U.S. institution of higher education; and
- Submit a Notice of Professional Public Service Activity Form for any financial interest in any amount received from a foreign person or entity (governmental, non-profit, for-profit, etc.).
A faculty member holding a full-time, regular appointment at UAB may not hold a regular teaching, training, or research appointment at another academic institution or research institute. As such, participation in talent programs, recruitment plans, visiting professorships, etc., in which ongoing responsibilities or services of teaching, training, or research duplicate or overlap those at UAB ENTERPRISE is prohibited. This prohibition does not include:
- joint programs between UAB ENTERPRISE and other institutions;
- Veteran’s Administration or Children’s of Alabama appointments;
- appointments arranged through approved sabbatical plans; or
- temporary appointments at previous employers to conclude prior mentoring or research service, which are acknowledged and approved in an official UAB ENTERPRISE appointment letter or other official transactions at the time of recruitment.
Other non-regular faculty appointments (e.g., adjunct) at other institutions are external activities, and as such, must be approved in advance as described above. This includes seasonal engagements UAB faculty with nine-month appointments may wish to undertake during summer terms or academic breaks. In considering requests for sabbatical and/or external activities at other academic institutions or research institutes, as part of the review process, chairs and deans must evaluate the nature of the activities, the volume and frequency of the time commitment required, and whether the activities are in competition with those at UAB or otherwise compromise or could be perceived to compromise the employee’s duty of loyalty to UAB. Copies of employment contracts or offer/appointment letters must accompany employees’ requests for approval.
Table. Differentiation of Professional Public Service Activities (PPSA) from External Activities (EA)
| If the activity is: | It is a PPSA if provided to: | It is an EA if: |
| • U.S. federal, state, or local government agencies • Institutions of higher education, academic teaching hospitals, medical centers or research institutes that are affiliated with an institution of higher education, whether U.S. or abroad • UAB Enterprise affiliated entities • Nonprofit/ philanthropic entities • Professional societies, or professional associations, that are not affiliates of or affiliated with industry or other for profit entities • Civic groups | Provided to anyone else |
| External employment including moonlighting and/or locum tenens | n/a | All |
| Consulting for any type of organization | n/a | All |
| Establishing and/or supporting a start-up company | n/a | All |
| Serving as an expert witness | n/a | All |
| Academic appointment, title, or other affiliation with another institution of higher education, domestic or foreign (only as permitted above) | n/a | All |
Figure. Flow of activities, disclosures, and leave requests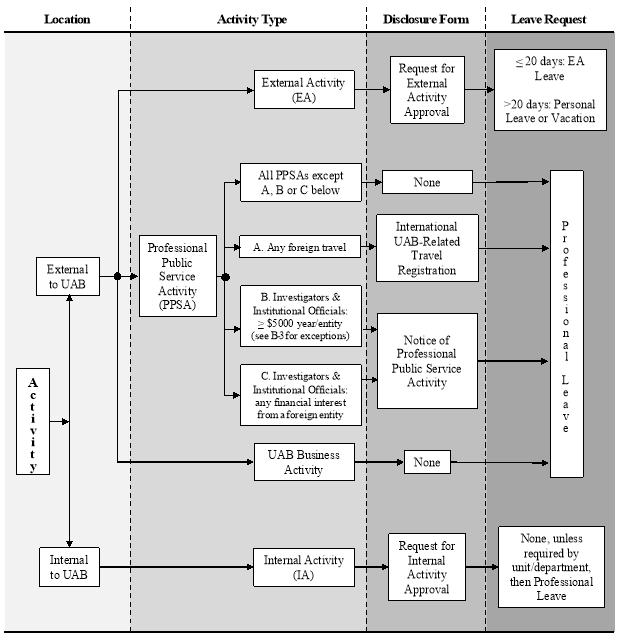
C. DISCLOSURE OF PERSONAL RELATIONSHIPS
Employees must fulfill their institutional responsibilities based on sound professional judgment unimpaired by their personal relationships. When participating in or influencing an official UAB ENTERPRISE decision (e.g., purchase of equipment, UAB ENTERPRISE contract for service with an external entity, approval or administrative control of research, etc.), employees are required to notify their chair, administrator, or supervisor and any relevant committee of any personal relationships that may influence or may be perceived to influence that decision. Such notification of personal relationships must take place prior to any official action. Appropriate action may be taken to manage the actual, potential, or perceived conflict of interest between the employees’ institutional responsibilities in this regard and their personal relationships, ranging from recusal from the decision to avoiding the transaction altogether.
D. DISCLOSURE OF FINANCIAL INTERESTS
Full-time faculty, investigators, and institutional officials are required to:
- submit formal disclosures of financial interests related to their institutional responsibilities;
- that are owned or held by them, their spouse, or their dependents;
- at the time they are appointed or otherwise so designated (in the case of investigators, prior to the application for research) and within 30 days of discovering or acquiring new financial interests;
- review and recertify any such disclosures of financial interests annually while maintaining such roles; and
- resolve through appropriate forms and channels any undisclosed financial interest, including any unapproved external activity or unnoticed qualifying professional public service activity, within 30 days of request for annual review.
Approved external activities and notices of professional public service activities are considered to be a disclosure of financial interest when the amount of remuneration from any activity or multiple activities for any one entity in the current and prior calendar year reaches the significant financial interest thresholds defined in this policy.
Annual disclosures within the UAB ENTERPRISE must not be confused with the annual Statement of Economic Interests required of certain public employees within the University by the Alabama Ethics Commission.
E. CONFLICTS OF INTEREST IN RESEARCH
UAB ENTERPRISE is committed to promoting objectivity in research and expects that the design, conduct, and reporting of research will be free from bias resulting from financial interests held by investigators and institutional officials.
Investigators are expected to comply with any applicable laws, regulations, and UAB ENTERPRISE policies pertaining to financial conflicts of interest. For all projects seeking or receiving extramural funding (including any grant/contract application processed by the UAB Office of Sponsored Programs (OSP)) and all work involving human subjects requiring Institutional Review Board review, regardless of funding source, participating UAB investigators are expected to comply with UAB policy, UAB Conflict of Interest Review Board (CIRB) rules, and the NIH rule “Responsibility of Applicants for Promoting Objectivity in Research for which Public Health Service Funding is Sought and Responsible Prospective Contractors” (42 CFR Part 50 and 45 CFR part 94). This includes, but is not limited to, the timely disclosure of financial interests, completing requisite training, and, as applicable, compliance with the Conflict of Interest Review Board (CIRB) or Institutional Conflict of Interest (ICOI) Committee rules and management plans.
The CIRB is responsible for reviewing investigators’ disclosed financial interests meeting the criteria (i.e., thresholds and types) for significant financial interests for the current and past calendar year to determine if any such interests are financial conflicts of interest related to research. The CIRB is required by regulation, policy, and as applicable, contractual obligation, to manage and report to funding agencies identified financial conflicts of interest. Management of an identified financial conflict of interest requires development and implementation of a management plan, which may include, but is not limited to, reducing or eliminating the financial conflict of interest. Whenever a financial conflict of interest is not identified or managed in a timely manner due to nondisclosure by the investigator, lack of institutional review or management of the financial conflict of interest, or failure by the investigator to comply with a management plan, the CIRB must conduct a retrospective non-compliance review and institute corrective actions as indicated by federal regulation. The CIRB must review significant financial interests related to the research of external consultants, subgrantees, and subcontractors that do not have policies meeting NIH regulations.
The CIRB is also responsible for reviewing institutional financial interests and institutional officials’ disclosures of financial interest to determine if any such interests are institutional conflicts of interest (ICOI) related to research. Reviews consider whether reported financial and/or fiduciary interests have the potential or appear to affect the safety of human research subjects or the integrity of research. The CIRB is responsible for managing institutional conflicts of interests in research that does not meet the Federal Drug Administration (FDA) definition of clinical investigation and that has been determined to be minimal risk to human participants by the reviewing IRB.
ICOIs related to research meeting the Federal Drug Administration (FDA) definition of clinical investigation and that has been determined to be greater than minimal risk to human participants by the reviewing IRB are referred for review to the ICOI Committee. All other matters related to ICOIs, including those involving minimal risk studies, will be reviewed and managed by the Office of the CIRB. The ICOI Committee is appointed by the University President and consists of seven members, three of which must be considered independent, having no financial relationship with UAB ENTERPRISE and not being influenced by interests of UAB ENTERPRISE.
In cases of research meeting the FDA definition of clinical investigation that has been determined to be greater than minimal risk by the reviewing IRB, the ICOI Committee reviews the ICOI to determine whether, and if so, how, the ICOI related to such research may be managed and forwards recommendations to the University President, who determines the outcome. The IRB of record has final authority to decide whether the financial or fiduciary interest and the management of the resulting institutional conflict of interest, if any, allows the clinical trial to be approved for implementation at UAB ENTERPRISE.
The standards and evaluation criteria must not vary by funding or regulatory oversight and equally apply to both institutional financial interests and the financial and/or fiduciary interests held by institutional officials. In all reviews, the CIRB and the ICOI Committee must retain documentation of the review in accordance with established guidelines.
F. CONFLICTS OF INTEREST IN EDUCATION
UAB ENTERPRISE must maintain an open academic culture, with decisions and actions related to education free of conflicts of interest related to any commercial interest. Commercial support of educational events, programs, or other activities sponsored by UAB must not influence educational content, research, or other scholarly activities. Employees must follow applicable contracting policies and unit-based standards for relationships with industry.
Faculty must adhere to the Authorship Policy before requiring for purchase in their courses or programs textbooks or other learning materials from which they may earn royalties. That policy seeks to balance the University’s ethical commitments to address any real or perceived conflict of interest with faculty’s academic freedom in this area.
- Solicitation or acceptance of personal gifts, food/beverages, services, gratuities, or other things of value by UAB ENTERPRISE employees, their spouses, or their dependents is prohibited if such solicitation or acceptance influences, or has the appearance of influencing, education, research, purchasing, or other official UAB ENTERPRISE business decisions.
- Gifts to University employees, their spouses, or their dependents are subject to further limitations defined within the Alabama Ethics Law, including de minimis value thresholds.
- UAB ENTERPRISE employees may accept awards and prizes from organizations and other entities outside the UAB ENTERPRISE for their teaching, research, and/or service performed in the course of their employment with UAB ENTERPRISE, provided that acceptance of such awards and prizes does not influence education, research, purchasing, or other official business decisions.
- Investigators, institutional officials, and full-time faculty may be required to disclose gifts, awards, or prizes meeting the stipulations of financial interests as described in Section D. above.
DISCIPLINARY ACTION
Confirmed violations of this policy will result in appropriate consequences commensurate with the offense, up to and including termination of employment, appointment, or other relationships with UAB ENTERPRISE.
IMPLEMENTATION
The Provost, Senior Vice President for Finance and Administration, and CEO of the UAB Health System are responsible for overall implementation of this policy. The Vice President for Research is responsible for the development and enforcement of procedures to implement the portions of this policy related to research.
DEFINITIONS OF ITALICIZED TERMS
The following defined terms are used for purposes of administering this policy but may be defined differently elsewhere in other UAB ENTERPRISE policies and materials.
Conflict of interest – a circumstance in which an individual’s financial, professional, or personal interests affect, or have the appearance of affecting, judgment in exercising a duty or responsibility owed to UAB ENTERPRISE. Financial conflict of interest related to the design, conduct, or reporting of research and institutional conflict of interest are defined separately below.
Conflict of commitment – a circumstance in which an employee’s engagement in external, internal, or professional public service activities compromises the ability to carry out his/her primary obligations and commitments to UAB ENTERPRISE. Because conflicts of commitment generally arise from allocation of time, the primary commitment of employees’ time should be toward their primary institutional responsibilities, as defined below.
Dependent – any individual, regardless of his or her legal residence or domicile, who receives 50% or more of his or her support from an investigator or institutional official or his/her spouse or who resided with the investigator or institutional official or his/her spouse for more than 180 days during a calendar year.
Employee – an individual who receives a W-2 from any UAB ENTERPRISE organization as a regular or temporary full-time employee, regular or temporary part-time employee, or irregular employee. For purposes of this policy, individuals receiving a W-2 as a student assistant, federal work study, graduate assistant, or credentialed credit course instructor are not included as employees, although they may be considered investigators if they are deemed responsible for the design, conduct, or reporting of research.
Entity – a company, association, organization, institution, or any other type of entity with a separate legal identity, including a for-profit, not-for-profit, or an organization of higher education. For purposes of reporting financial interests under this policy, it also includes an individual.
External activities – activities that draw upon the knowledge, skill, or abilities an employee uses to fulfill his or her institutional responsibilities at UAB ENTERPRISE and that are performed for an entity outside the UAB ENTERPRISE, whether foreign or domestic, and whether or not for compensation. Examples of external activities include, but are not limited to, the following:
- external employment, including moonlighting and/or locum tenens activities;
- consulting;
- lecturing, presenting, performing, or speaking;
- establishing and/or supporting a start-up company;
- serving as an expert witness;
- participating in a board of directors or similar governing body;
- participating in a scientific advisory board; or
- appointments or other commitments to other academic institutions or research institutes (as permitted above).
Financial conflict of interest – a reasonable determination made by the UAB CIRB and/or Office that an investigator’s or institutional official’s significant financial interest is related to and could significantly and directly affect the design, conduct, or reporting of research.
Financial interests – anything of monetary value accepted or owned by an full-time faculty member, investigator, institutional official, his/her spouse, or his/her dependents, not held in an investment vehicle such as a mutual fund or retirement account in which the owner does not directly control investment decisions, whether or not the value is readily ascertainable. Examples of financial interests include, but are not limited to:
- remuneration for participation in external activities (e.g., salary, consulting and other fees, gifts, honoraria, etc.);
- acquisition or ownership of (or an option to acquire or own) stock, shares, or other types of equity interests;
- income arising from stock, shares and other types of equity interests;
- income received from royalties (e.g., for sale by volume of products, textbooks, etc.);
- income received from commercialization of intellectual property (e.g., for licenses, options, or other revenue generating activity); or
- sponsored or reimbursed travel.
Institutional financial interest – financial interests held by the University (through the UAB Research Foundation) including:
- Equity and ownership interests worth more than $100,000 in any publicly traded, for-profit organization, except for interests held in the institution’s endowments, and those interests where institutional officials have no role in trading decisions;
- Gifts totaling $500,000 or more over the course of 12 months, whether one-time or installments, from any for-profit organization or philanthropic unit associated with a for-profit organization;
- Equity and ownership interests of any amount in any for-profit organization that is not publicly traded, except those interests where institutional officials have no role in trading decisions; and
- Arrangements with third parties to license or otherwise transfer UAB intellectual property (not including research tools or where the federal government has exercised march-in rights). Clinical care income and tuition income are not included as institutional financial interests.
Institutional conflict of interest (ICOI) – a circumstance in which institutional financial interests or the personal financial or fiduciary interests of institutional officials affect, or have the appearance of affecting, institutional processes for the design, conduct, reporting, review, or oversight of research. Specifically, an ICOI in research exists when the University or an institutional official holds an interest in:
- a potential commercial sponsor of research;
- a company that owns or controls products being studied or tested; or
- the intellectual property being studied or tested.
Institutional official – an employee with direct authority over allocation of institutional resources, assignments of graduate students, trainees, funding, space, salary, or promotions for faculty. For purposes of this policy, institutional officials include the University President; University Provost; University Vice Presidents; Deans; Chairs; and other administrators in key decision-making, signatory, or assurance roles, as determined and notified in writing by the Chief Risk & Compliance Officer in consultation with the University President.
Institutional responsibilities – all activities, duties, and responsibilities performed by an employee in the course of his/her employment or other relationship with UAB ENTERPRISE, including but not limited to scholarship, research, research consultation, teaching, professional practice, administration, contracting or procurement responsibilities, or professional public service activities.
Internal activities – activities that draw upon the knowledge, skill, or abilities an employee uses to fulfill his or her institutional responsibilities at UAB ENTERPRISE and that are performed for another UAB ENTERPRISE organization or another unit within the same organization in the UAB ENTERPRISE, the Veteran’s Administration, or Children’s Hospital of Alabama for a limited duration for compensation in addition to the salary and compensation attributable to the employee’s appointments or assignments at UAB ENTERPRISE. An internal activity does not include a secondary (or otherwise additional) appointment or assignment or other work project with another UAB ENTERPRISE organization, the Veteran’s Association, or Children’s of Alabama in which an employee’s time or effort is shared or which is part of an employee’s job expectations. In those instances, the organization receiving the services must make appropriate financial arrangements for payment and/or distribution of the portion of the employee’s salary attributable to that appointment, assignment, or other work project.
Investigator/Responsible personnel – any individual, regardless of title, position, or employment, who is responsible for the design, conduct, or reporting of proposed or active research.
Professional Public Service Activities (PPSA) – those activities specifically enumerated below for the groups specifically enumerated below, which are considered part of an employee’s institutional responsibilities, whether or not separately compensated:
- professional studies (e.g., attendance at scientific meetings);
- seminars, lectures, performances, presentations, or continuing education sessions;
- service on review panels (e.g., participation in manuscript review, grant/contract review, academic program review, etc.);
- service on advisory committees; or
- service on a Board of Directors or similar governing body.
provided to:
- U.S. federal, state, or local government agencies;
- institutions of higher education, academic teaching hospitals, medical centers, or research institutes affiliated with an institution of higher education, whether U.S. or abroad;
- nonprofit/philanthropic entities, professional societies, or professional associations, that are not affiliates of or affiliated with industry or other for profit entities;
- organizations accredited or approved by the appropriate independent boards or bodies governing oversight of continuing professional education activities; or
- civic groups.
Research – a systematic investigation or inquiry adding to the general body or application of knowledge. For purposes of this policy, research additionally regards any activity for which funding is sought and/or received through a grant or cooperative agreement, regardless of funding source, such as a research grant, career development award, center grant, individual fellowship award, contract, infrastructure award (construction, renovation, equipment, etc.) institutional training grant, program project, or research resources award. The term also includes all work involving human subjects requiring Institutional Review Board approval.
Responsible for the design, conduct, or reporting of research – an investigator is responsible for the design, conduct, or reporting of research as follows:
- Design – the development of the strategy and means to test a research question or hypothesis.
- Conduct – the supervision or management of a study’s execution. This is typically done by the principal investigator (PI) and co-investigators, but also may be performed by other project personnel, such as postdoctoral fellows, graduate students, or other junior researchers. For studies involving human subjects, this includes anyone who is responsible for explaining the study, risk -benefit, and/or alternatives to potential participants, is listed on Form FDA-1572 or device agreement, and/or must complete a sponsor’s conflict of interest form.
- Reporting – the authorship of publications or reports that describe the results of the study. Such reports may be to the sponsor of the research or to academic or scientific meetings.
Significant financial interest (SFI) – thresholds (i.e., types and amounts) of financial interest prompting the UAB Office of the Conflict of Interest Review Board to conduct review for relatedness to research and financial conflicts of interest.
- With regard to any publicly-traded entity, a significant financial interest exists if the value of the financial interest received from the entity in the current or prior calendar year, when aggregated, exceeds $5,000.
- With regard to any non-publicly traded entity, a significant financial interest exists if:
- the value of any remuneration received from the entity in the current or prior calendar year, when aggregated, exceeds $5,000; or
- when the investigator or institutional official (or the investigator’s or institutional official’s spouse or dependents) holds any equity interest (e.g., stock, stock option, or other ownership interest).
- With regard to intellectual property rights and interests (e.g., patents, copyrights), a significant financial interest exists for any intellectual property licensed, optioned, or that has generated income/revenue.
- Significant financial interest does NOT include remuneration for PPSAs with U.S. entities, textbook royalties, peer reviewed journal editorship activities for publishing companies, or other related items as determined by the CIRB in accordance with federal regulations.
UAB ENTERPRISE – the following organizations:
- UAB
- UAB Hospital - Callahan Eye
- UAB Health System
- UAB Hospital Management, LLC
- University of Alabama Health Services Foundation, P.C.
- University of Alabama Ophthalmology Services Foundation
- Triton Health System, LLC
- Valley Foundation
- VIVA Health, Inc.
- VIVA Health Administration, LLC.
Related Policies
Employment and Other External Activities - HR Policy 128
Consulting / Independent Contractors - HR Policy 127
Fundraising Policy and Gift Acceptance Guidelines
Internal Consulting by UAB Employees - HR Policy 129
Nepotism - HR Policy 123Internal Consulting by UAB Employees - HR Policy 129
Nepotism - HR Policy 123